There’s something incredibly satisfying about watching an ultrasonic cleaner work. That quiet hum, the bubbling action, the cloudy water slowly revealing a sparkling-clean item beneath. It’s a small moment of magic—science and practicality wrapped in one.
And naturally, if you’re like many home users, you’ve probably wondered: can I just throw in some baking soda instead of buying fancy detergent? It’s cheap, safe, natural. You use it in the kitchen, in the laundry, even for brushing your teeth. So why not in your ultrasonic cleaner?
But here’s where curiosity and chemistry start to clash. While baking soda might seem like a harmless, even genius addition, the truth is a little more complicated—and a lot more interesting.
Why People Consider Baking Soda in Ultrasonic Cleaners
First, let’s acknowledge the logic. Baking soda (sodium bicarbonate) has long been a household cleaning hero. It’s mildly alkaline, deodorizes without being harsh, and scrubs without scratching—at least on most surfaces. Add in the non-toxic, environmentally friendly factor, and it makes perfect sense that people want to extend its magic into ultrasonic cleaning.
You’ll find plenty of anecdotal advice online—from DIYers cleaning coins to denture wearers soaking aligners—all swearing by a pinch of baking soda in their tanks. But just because it seems to work doesn’t always mean it’s ideal.
How Ultrasonic Cleaners Actually Work
Ultrasonic cleaners don’t rely on chemical abrasives or scrubbing action like traditional cleaning. Instead, they generate high-frequency sound waves (typically 20,000–45,000 Hz) through a transducer that creates microscopic bubbles in the cleaning solution. This is called cavitation—a cycle of formation and collapse of vacuum bubbles.
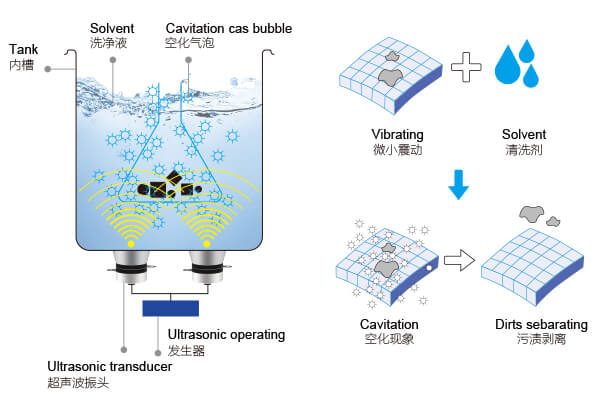
As these bubbles implode near an item’s surface, they dislodge grime, oils, and contaminants—even from the tiniest crevices. It’s incredibly efficient, especially when paired with the right cleaning chemistry.
Which brings us to the big question: What happens when that chemistry includes baking soda?
Chemical Behavior of Baking Soda in Ultrasonic Baths
Baking soda is mildly alkaline (with a pH of ~8.3 when dissolved in water) and very slightly abrasive in its powdered form. When added to warm water in small amounts, it dissolves reasonably well. But it’s not a surfactant. It won’t lower surface tension, break down oils, or emulsify grime the way a formulated detergent would.
In an ultrasonic bath, it can do one of three things:
- Act as a weak deodorizer and mild alkali, helping to soften certain residues.
- Produce micro-abrasive effects if not fully dissolved, potentially damaging softer surfaces.
- Create sediment or cloudiness in the water, especially if too much is added or the water is not agitated enough.
It won’t foam or damage the machine directly—but it also won’t do much to support cavitation or cleaning efficiency.
Material Safety—What Can and Can’t Be Cleaned With Baking Soda in an Ultrasonic Bath
Let’s get practical. What are you trying to clean?
For hard metals like stainless steel or silver, baking soda is relatively safe—though not ideal. It may leave behind fine residue or dull finishes. For softer materials like gold, aluminum, plastic, or coated parts (like anodized metals), the risk goes up.
Why? Because any undissolved particles or cumulative residue can act like a slurry under vibration, potentially causing micro-scratches or discoloration.
Abrasiveness and Surface Damage Risks
Baking soda is often described as “non-scratch,” but that’s only in the context of hand cleaning. In an ultrasonic bath, where particles are bouncing and cavitating under intense pressure, even a soft abrasive can become damaging—especially to soft gemstones, plastics, or delicate dental devices.
Residue Build-Up and Internal Component Risk
Another concern is sediment buildup. Unlike true ultrasonic detergents, baking soda doesn’t fully suspend particles or oils. This means over time, you may see white deposits forming at the bottom of your tank—or worse, in pumps and filters (if your cleaner has a recirculating system).
Common Misconceptions Debunked
Let’s tackle a few popular myths:
- “It’s natural, so it must be safe.”
→ Nature doesn’t equal universal safety—especially with ultrasonic vibration. - “It’s cheaper than detergent, so why not?”
→ True, but cost-saving doesn’t mean effective cleaning. It may just result in repeated cycles or partial results. - “My grandma used it for everything.”
→ She probably didn’t own an ultrasonic cleaner with a high-frequency piezoelectric transducer.
Expert and Manufacturer Recommendations
Most professional ultrasonic cleaner manufacturers recommend against using baking soda in their machines. Why? It’s not designed for cavitation efficiency, and it leaves behind residue that can affect performance over time.
Instead, they recommend using cleaners like:
- Elma Clean EC 55: For metals and general cleaning
- Branson GP or MC-3: Multi-purpose formulas with enzymatic and degreasing properties
- iSonic Cleaning Concentrate CSGJ01: For jewelry, aligners, dentures
These are formulated to enhance cavitation while being safe for tanks and components.
Here’s the revised Real-World User Experiences section with actual quotes and direct citations from authoritative sources:
Real‑World User Experiences: What Forums and Reviews Reveal
Brushing past marketing claims, let’s look at what real people are saying in the field—posts from Reddit and enthusiast forums where users share honest test results and practical advice.
r/Invisalign community
One user expressed disappointment comparing baking soda to ultrasonic cleaning:
“Honestly, I was disappointed today when I tried the ultrasonic cleaner and it didn’t make them look any better than the baking soda did.” (Corresponding links:https://www.reddit.com/r/Invisalign/comments/si4pr5/ultrasonic_cleaner/, https://www.reddit.com/r/Invisalign/comments/1im867c/cleaning_retainers_with_baking_sodabaking_powder/)
This suggests baking soda alone may work similarly to ultrasonic, but doesn’t necessarily improve cleaning results—with the added hassle and risk.
r/BuyItForLife — Dental appliance advice
A community member shared caution over using baking soda:
“Baking soda works, but is abrasive and should be done very sparingly.” (Corresponding links:https://www.reddit.com/r/BuyItForLife/comments/1fb3vdk/a_good_ultrasonic_cleaner_for_teeth_retainers/)
That echoes broader concerns—baking soda might clean, but at what cost to materials?
ScubaBase & Hunter forums
Users who clean metal parts or brass with ultrasonics often neutralize acidic solutions using baking soda afterward:
“Works well. Once cleaned I run everything in baking soda to neutralise the citric acid.” (Corresponding links:https://www.snipershide.com/shooting/threads/ultrasonic-cleaners.6977908/)
These aren’t endorsements for ongoing use—but reminders that baking soda is useful as a neutralizer after acid-based cleaning, not as a standalone detergent.
What We Learn from Real Experiences
From these firsthand accounts, patterns emerge that echo our chemical understanding:
- Baking soda alone won’t enhance ultrasonic cleaning—and may offer no visual benefit over plain water.
- It is abrasive enough to warrant caution, especially with delicate items.
- In industrial workflows, baking soda often plays a maintenance role—not the main cleaning agent.
Together, this real-world feedback supports the conclusion: baking soda can be used sparingly and strategically, but it should not replace proper ultrasonic detergents.
Can You Use Baking Soda in a Pinch? If Yes, How?
Now, let’s not be dramatic. If you’ve already tried baking soda in your ultrasonic cleaner or you’re in a tight spot with no access to proper detergent, you’re not doomed. There are scenarios where using a very small amount of baking soda—carefully and temporarily—may still yield acceptable results.
For example, if you’re cleaning stainless steel tools, coins, or items that don’t have delicate coatings or soft materials, a ¼ teaspoon of baking soda per liter of warm water, thoroughly dissolved, can provide mild deodorizing without noticeable damage.
The rules of thumb?
- Use baking soda only in low concentrations
- Dissolve it completely in water before activating the cleaner
- Avoid using it regularly—think emergency use, not routine maintenance
- Always rinse items thoroughly and wipe the tank after use
It’s like using vinegar to wipe a screen—not a long-term solution, but it works if you’re out of wipes.
How to Clean and Maintain the Ultrasonic Cleaner After Using Baking Soda
If you’ve used baking soda—even just once—it’s smart to give your machine a little TLC. Ultrasonic cleaners rely on consistent transducer performance and tank cleanliness to function optimally.
Here’s a quick post-clean checklist:
- Drain the tank and flush it with warm water.
- Wipe the sides and bottom with a soft cloth to remove any lingering residue.
- Run a quick cycle with plain water to ensure the transducer is clean.
- Avoid letting any water sit in the tank between uses—dry thoroughly.
- If your cleaner has a filter or pump system, check for sediment and rinse thoroughly.
For heavy buildup (from repeated soda use or hard water), a diluted white vinegar rinse (1:5 ratio) followed by plain water rinse can help descale the tank.
DIY vs Pro-Grade Cleaning: Why It Matters
Let’s say you’re cleaning something inexpensive and sturdy—maybe a few old bolts, a razor head, or your bicycle’s derailleur. You might get by with baking soda. It’s cost-effective, available, and decently functional for quick, light cleans.
But if you’re cleaning:
- Delicate optical lenses
- Dental retainers or aligners
- Expensive jewelry
- Watch components
- Brass musical instruments
…then the risks quickly outweigh the savings. Precision cleaning requires precision chemistry. The wrong solution might cloud your lens, scratch your diamond, or leave residues that dull metal over time.
Professionals spend good money on ultrasonic cleaning solutions not just for power—but for control, safety, and repeatable results. When accuracy matters, DIY shortcuts start to lose their appeal.
Final Take: When Is It OK to Use Baking Soda, and When Should You Avoid It?
So, can you use baking soda in an ultrasonic cleaner?
Yes—with caveats. It’s okay for basic cleaning of tough metal objects or simple surfaces, used sparingly and occasionally. But it’s not recommended for daily use, soft materials, delicate surfaces, or anything with coatings, elastomers, or intricate mechanics.
When in doubt, opt for a proper ultrasonic cleaning solution. These are designed to optimize cavitation, maintain your machine’s longevity, and protect your items—without foaming, scratching, or leaving mystery residues behind.
In a world where your tools, glasses, or dental appliances cost far more than a $10 bottle of cleaner, the investment in proper chemistry just makes sense.
FAQs
1. Can baking soda damage jewelry in an ultrasonic cleaner?
Yes—especially gold-plated or soft gemstones. Abrasive particles may scratch surfaces or leave dull residue.
2. Is it safe to clean retainers or aligners with baking soda ultrasonically?
Not ideal. Baking soda can cloud plastic, and without surfactants, it won’t effectively break down biofilm.
3. Will baking soda harm the ultrasonic cleaner itself?
Not directly, but it can leave residue that affects cleaning performance or clogs pump systems over time.
4. Can I mix baking soda with other cleaners?
No. It may react with acidic or foaming agents, destabilizing the solution and harming your items.
5. What’s a better alternative to baking soda in ultrasonic cleaners?
Use enzyme-based or alkaline ultrasonic cleaning solutions specifically designed for your item—e.g., iSonic, Elma Clean, Branson GP.
6. Does baking soda enhance cavitation?
No. It doesn’t improve bubble action or surfactant behavior—in fact, it may slightly hinder cavitation if not fully dissolved.